
These cycles are called chemical responses and, by and large, are not reversible besides by additional chemical responses. Albeit compound changes might be perceived by a sign, for example, scent, shading change, or creation of gas, all of these pointers can result from the physical change.Ī chemical change is said to happen when one chemical substance is transformed into one or more different substances, or chemical changes happen when a substance consolidates with one or more to shape another substance, called the chemical union, or, on the other hand, chemical decay into at least two distinct substances. Numerous substance changes are irreversible, and numerous physical changes are reversible however, reversibility is anything but a specific standard for order. Numerous physical changes additionally include the adjustment of molecules most discernibly in the development of precious stones.

A steel clear is over and again warmed and pounded which changes the hardness of the steel, its adaptability, and its capacity to keep a sharp edge. Instances of physical properties incorporate softening, progress to a gas, change of solidarity, change of sturdiness, changes to gem structure, textural change, shape, size, shading, volume, and thickness.Īn illustration of a physical change is the most common way of treating steel to frame a blade sharp edge. For instance, salt dissolved in water can be recuperated by permitting the water to vanish.Ī physical change includes a change in physical properties.

Overall a physical change is reversible utilizing physical means. This differentiates with the idea of synthetic change wherein the structure of a substance changes or at least one substance consolidates or separated to shape new substances. Physical changes happen when articles or substances go through a change that doesn't change their compound creation. Physical changes are utilized to isolate blends into their part compounds yet can not typically be utilized to isolate compounds into synthetic components. Physical changes are the changes that influence the type of a synthetic substance, yet not its compound creation. Examples of physical changes: boiling water, breaking a glass, melting an ice cube, freezing water, mixing sand and water, crumpling of paper, and melting a sugar cube. Physical changes only change the appearance of a substance, not the chemical composition. A change in which a substance undergoes a change in its physical properties is termed physical change. Properties such as shape, size, volume, colour, appearance, and state of a substance (solid, liquid, and gas) are called physical properties.
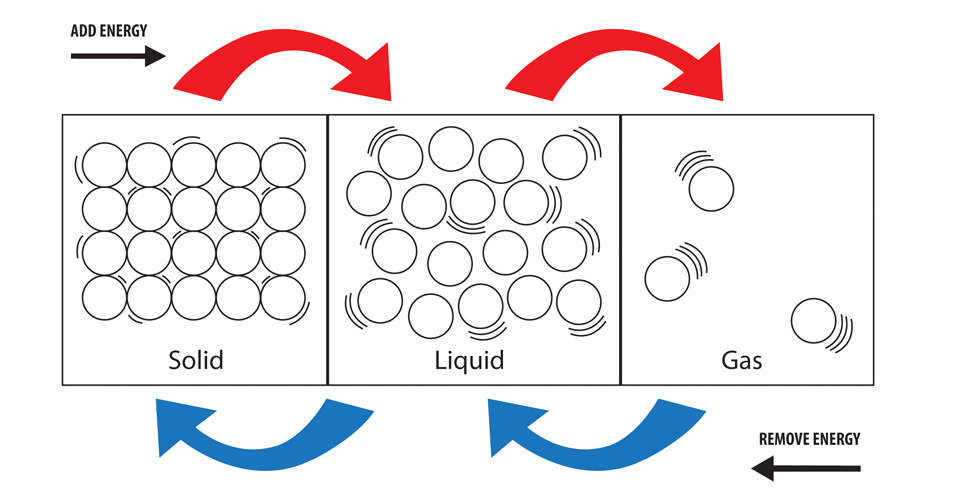
We will understand more about this by starting with the physical change definition. There are many reasons for these physical and chemical changes. Have you ever tried to understand these changes? We should try to understand the terms that are physical and chemical changes and examples, reversible and irreversible changes before knowing the scientific reason for changes. Unexpected color changes (a substance with a different color is made, rather than just mixing the original colors together).We see different types of physical and chemical changes in our surroundings like dissolving sugar and water, burning of coal, rusting, melting an ice cube, boiling water, different shape and size of the Moon, etc., change is occurring all around us every time.Temperature changes (either the temperature increases or decreases).Observations that help to indicate chemical change include:

Some of the evidence for chemical change will involve the energy changes that occur in chemical changes, but some evidence involves the fact that new substances with different properties are formed in a chemical change. We have to make other observations to indicate that a chemical change has happened. We can't actually see molecules breaking and forming bonds, although that's what defines chemical changes. \): Burning of wax to generate water and carbon dioxide is a chemical reaction.


 0 kommentar(er)
0 kommentar(er)
